- Contact Us
- News and Events
- Investor Relations
- Locations and Facilities
- Careers
- About Our Site
- Accessibility Statement
- Privacy Policy
- Terms and Conditions
- For Employers and Medical Plan Providers
- For Pharmacists and Medical Professionals
- Drug Lists
- Drug Safety Alerts
- Emergency Response
- Mail Service Numbers, Forms and Information
- Specialty Pharmacy Services, Information and Forms
- Electronic Prior Authorization Information
- Clinical Drug Information
- Clinical Programs and Health Management
- Clinical Publications
- FAQs For Pharmacists and Pharmacy Staff
- Pharmacy Help Desk
- FAQs For Prescribers and Office Staff
- Contact Us | Health Professionals Only
Drug Safety Alerts
Losartan Potassium 25 mg and 50 mg Tablets manufactured by Torrent Pharmaceuticals Limited that were repackaged and distributed by AvKare - Expanded Consumer-level Recall
04/24/2019
On April 24, 2019, AvKare issued an expanded consumer-level recall of Losartan Potassium 25 mg and 50 mg Tablets manufactured by Torrent Pharmaceuticals Limited that were repackaged and distributed by AvKare. This recall was issued due to the detection of trace amounts of an unexpected impurity found in an active pharmaceutical ingredient (API) manufactured by Hetero Labs Limited. The impurity detected in the API is N-Methylnitrosobutyric acid (NMBA).
This recall affects the following product and lot number:
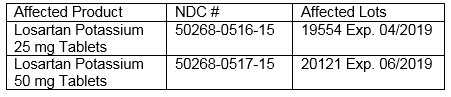
Losartan is used to treat hypertension, hypertensive patients with Left Ventricular Hypertrophy and for the treatment of nephropathy in Type 2 diabetic patients.
Patients who are on losartan potassium tablets should continue taking their medication, as the risk of harm to the patient’s health may be higher if the treatment is stopped immediately without any alternative treatment. Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication.
Note: This recall was originally issued on March 12, 2019. The expanded recall notice on April 24, 2019 now includes Losartan Potassium 25 mg Tablets and one more lot of Losartan Potassium 50 mg Tablets that were not part of the original recall notice.
CVS Caremark Response: CVS Caremark Mail Service Pharmacy does not carry Losartan Potassium 25 mg and 50 mg Tablets repackaged and distributed by AvKare.
For more information about the recall, please call AvKare toll-free at 1‑855‑628-5273 (1‑855‑6AV-KARE). You may also call the U.S. Food and Drug Administration (FDA) consumer inquiry line at 1-888-INFO-FDA (1-888-463-6332) or visit www.fda.gov.