- Contact Us
- News and Events
- Investor Relations
- Locations and Facilities
- Careers
- About Our Site
- Accessibility Statement
- Privacy Policy
- Terms and Conditions
- For Employers and Medical Plan Providers
- For Pharmacists and Medical Professionals
- Drug Lists
- Drug Safety Alerts
- Emergency Response
- Mail Service Numbers, Forms and Information
- Specialty Pharmacy Services, Information and Forms
- Electronic Prior Authorization Information
- Clinical Drug Information
- Clinical Programs and Health Management
- Clinical Publications
- FAQs For Pharmacists and Pharmacy Staff
- Pharmacy Help Desk
- FAQs For Prescribers and Office Staff
- Contact Us | Health Professionals Only
Drug Safety Alerts
Irbesartan and Irbesartan/hydrochlorothiazide (HCTZ) Tablets - Consumer-level Recall
01/18/19
On January 18, 2019, Solco Healthcare U.S. issued a consumer-level recall of irbesartan and irbesartan/hydrochlorothiazide (HCTZ) tablets. This recall was issued due to the detection of a trace amount of an unexpected impurity, N-nitrosodiethylamine (NDEA), made by the manufacturer – Zhejiang Huahai Pharmaceutical Co. Ltd.-- that is used in the manufacture of the subject product lots. This impurity has been classified as a probable human carcinogen as per the International Agency for Research on Cancer (IARC) classification. To date, Solco Healthcare has not received any reports of adverse events related to this recall.
Irbesartan and irbesartan/hydrochlorothiazide (HCTZ) are used to control high blood pressure and for the treatment of heart failure. Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medications.
Please note: The United States Food and Drug Administration (FDA) recommends that patients should continue taking their medication until their pharmacist provides a replacement, or their doctor prescribes a different medication that treats the same condition. The risk of harm to a patient’s health may be higher if the treatment is stopped immediately without any alternative treatment.
This recall affects the following products and lot numbers:
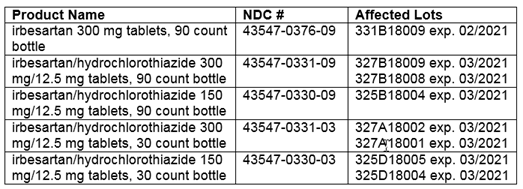
CVS Caremark Response: Because of this action, CVS Caremark Mail Service Pharmacy is contacting patients and prescribers to advise them of this recall. We are encouraging patients who are taking irbesartan and irbesartan/hydrochlorothiazide (HCTZ) to contact their prescriber.
For more information about this recall, please call Solco Healthcare U.S. toll-free at 1‑866‑931‑ 9829. You may also call the U.S. Food and Drug Administration (FDA) consumer inquiry line at 1-888-INFO-FDA (1-888-463-6332) or visit www.fda.gov.