- Contact Us
- News and Events
- Investor Relations
- Locations and Facilities
- Careers
- About Our Site
- Accessibility Statement
- Privacy Policy
- Terms and Conditions
- For Employers and Medical Plan Providers
- For Pharmacists and Medical Professionals
- Drug Lists
- Drug Safety Alerts
- Emergency Response
- Mail Service Numbers, Forms and Information
- Specialty Pharmacy Services, Information and Forms
- Electronic Prior Authorization Information
- Clinical Drug Information
- Clinical Programs and Health Management
- Clinical Publications
- FAQs For Pharmacists and Pharmacy Staff
- Pharmacy Help Desk
- FAQs For Prescribers and Office Staff
- Contact Us | Health Professionals Only
Drug Safety Alerts
Vecuronium bromide for injection - Class I Recall
01/16/2019
On January 16, 2019, the United States Food and Drug Administration (FDA) issued a notice regarding a Class I recall of vecuronium bromide for injection manufactured by Sun Pharmaceuticals. This recall was issued because foreign matter identified as glass was detected in one (1) vial.
The administration of a glass particulate, if present in an intravenous drug, may result in local irritation or swelling in response to the foreign material. More serious potential outcomes would include blockage and clotting in blood vessels, which may be life-threatening. To date, Sun Pharmaceuticals has not received any reports of adverse events related to this recall.
Vecuronium Bromide for Injection is used as an adjunct to general anesthesia, to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation and is packaged in a glass vial; ten vials per carton. Vecuronium Bromide for Injection should be administered by or under the supervision of experienced clinicians and must be reconstituted prior to use.
This recall was originally received as retail-level recall on January 7, 2019. The FDA classified this as a Class I Recall on January 16, 2018.
This recall affects the following products and lot numbers:
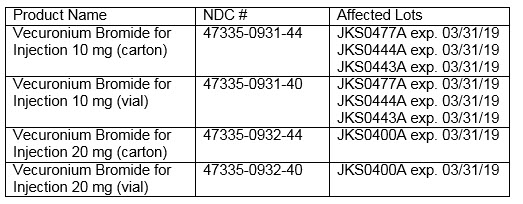
CVS Caremark Response: This product is not carried by the CVS Caremark Mail Service Pharmacies.
For more information, please call Sun Pharmaceuticals Drug Safety Information toll-free at 1‑800‑406-7984, Monday through Friday, 8:00 am to 5:00 pm (ET). You may also call the FDA consumer inquiry line toll-free at 1‑888‑INFO-FDA (1‑888-463-6332) or visit https://www.accessdata.fda.gov.